MyMD Pharmaceuticals Announces Issuance of Allowance from United States Patent and Trademark Office for Synthetic Cannabinoid Compounds
- Patent protects Supera-CBD, a preclinical cannabidiol derivative that targets cannabinoid receptor type 2 for the treatment of neuroinflammatory and neurodegenerative diseases.
BALTIMORE--(BUSINESS WIRE)-- MyMD Pharmaceuticals, Inc. (Nasdaq: MYMD), a clinical stage pharmaceutical company committed to extending healthy lifespan by focusing on developing two therapeutic platforms, announced today that the United States Patent and Trademark Office (USPTO) issued a Notice of Allowance for application no. 16/612,472, entitled “Synthetic Cannabinoid Compounds for the Treatment of Substance Addiction and Other Disorders.”
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210422005689/en/
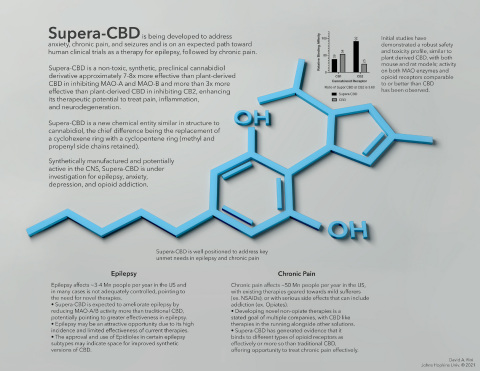
Supera-CBD is well positioned to address key unmet needs in epilepsy and chronic pain (Graphic: Business Wire)
The allowed claims cover the new molecular entity Supera-CBD as well as pharmaceutical compositions containing the compound. The USPTO found the claims patentable based in part on the compound’s unexpectedly improved activity and selectivity toward inhibiting cannabinoid receptor type 2 (CB2), a key therapeutic target for neuroinflammatory and neurodegenerative diseases.
“The issuance of this Notice of Allowance for our molecule, Supera-CBD, continues to demonstrate our steadfast commitment to moving forward with and protecting our entire product portfolio," said Adam Kaplin, M.D., Chief Scientific Officer of MyMD. “We are thrilled that Supera-CBD has shown strong promise in treating neuroinflammatory and neurodegenerative diseases, and is expected to be a major focus for our company as we move forward.”
Supera-CBD is a non-toxic, synthetic, preclinical cannabidiol derivative that has been shown in in vitro studies to be approximately 7-8x more effective than plant-derived CBD in inhibiting MAO-A and MAO-B and more than 3x more effective than plant-derived CBD in inhibiting CB2, which should enhance its therapeutic potential to treat pain, inflammation, and neurodegeneration.
Supera-CBD is being developed to address anxiety, chronic pain, and seizures and is on an expected path toward human clinical trials as a proposed therapy for epilepsy, followed by chronic pain.
MyMD has continued to elevate its work in inflammatory diseases and was previously issued US Patent Number 10,835,523 B2, titled “Method of Regulating Tumor Necrosis Factor-Alpha (TNF-α) for Treating Cancers, Autoimmune Disorders, and Other Disorders Associated with Chronic Inflammation,” which was a significant milestone in the company’s approach toward anti-aging product therapies.
The grant of the ‘472 application will add to the company’s growing worldwide patent portfolio, which includes eleven granted patents pertaining to its lead compound, MYMD-1. These patents, coupled with clinical studies underway, have laid the foundation for the planned Phase 2 trial investigating the use of MYMD-1 in patients with depression due to COVID-19, which was recently announced.
About MyMD Pharmaceuticals, Inc.
MyMD is a clinical stage pharmaceutical company committed to extending healthy lifespan by focusing on developing two therapeutic platforms. MYMD-1 is a drug platform based on a clinical stage small molecule that regulates the immunometabolic system to control TNF-α and other pro-inflammatory cytokines. MYMD-1 is being developed to treat autoimmune diseases, including those currently treated with non-selective TNF-α blocking drugs, and aging and longevity. Supera-CBD is a drug platform based on a novel (patent pending) synthetic derivative of cannabidiol (CBD) that targets numerous key receptors including CB2 and opioid receptors and inhibits monoamine oxidase. Supera-CBD is being developed to address the rapidly growing CBD market, that includes FDA approved drugs and CBD products not currently regulated as a drug. For more information, visit www.mymd.com.
Cautionary Statement Regarding Forward-Looking Statements
This press release may contain forward-looking statements. These forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results, performance or achievements to be materially different from any expected future results, performance, or achievements. Forward-looking statements speak only as of the date they are made and none of MyMD nor its affiliates assume any duty to update forward-looking statements. Words such as "anticipate," "believe," "could," "estimate," "expect," "may," "plan," "will," "would'' and other similar expressions are intended to identify these forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, without limitation: the timing of, and MyMD’s ability to, obtain and maintain regulatory approvals for clinical trials of MyMD’s pharmaceutical candidates, the timing and results of MyMD’s planned clinical trials for its pharmaceutical candidates, the amount of funds MyMD requires for its pharmaceutical candidates; increased levels of competition; changes in political, economic or regulatory conditions generally and in the markets in which MyMD operates; MyMD’s ability to retain and attract senior management and other key employees; MyMD’s ability to quickly and effectively respond to new technological developments; MyMD’s ability to protect its trade secrets or other proprietary rights, operate without infringing upon the proprietary rights of others and prevent others from infringing on MyMD’s proprietary rights; and the impact of the ongoing COVID-19 pandemic on MyMD’s results of operations, business plan and the global economy. A discussion of these and other factors with respect to MyMD is set forth in the registration statement on Form S-4 filed by MyMD on January 15, 2021, as amended. Forward-looking statements speak only as of the date they are made and MyMD disclaims any intention or obligation to revise any forward-looking statements, whether as a result of new information, future events or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210422005689/en/
Investor Contact:
Brett Mass
646-536-7331
brett@haydenir.com
www.haydenir.com
Media Contact:
Will Johnson
201-465-8019
MYMD@antennagroup.com
www.antennagroup.com
Source: MyMD Pharmaceuticals, Inc.
Released April 22, 2021